research groups
Structural Microbiology and Biodesign lab
summary

- Lisa, María Natalia Location: CCT
Email: lisa@ibr-conicet.gov.ar
- Chamorro, Nicolas
- Rapino, Carina
- Azzolini, Juan Manuel
RESEARCH LINES
Molecular mechanisms of nitrogen metabolism regulation in Actinobacteria
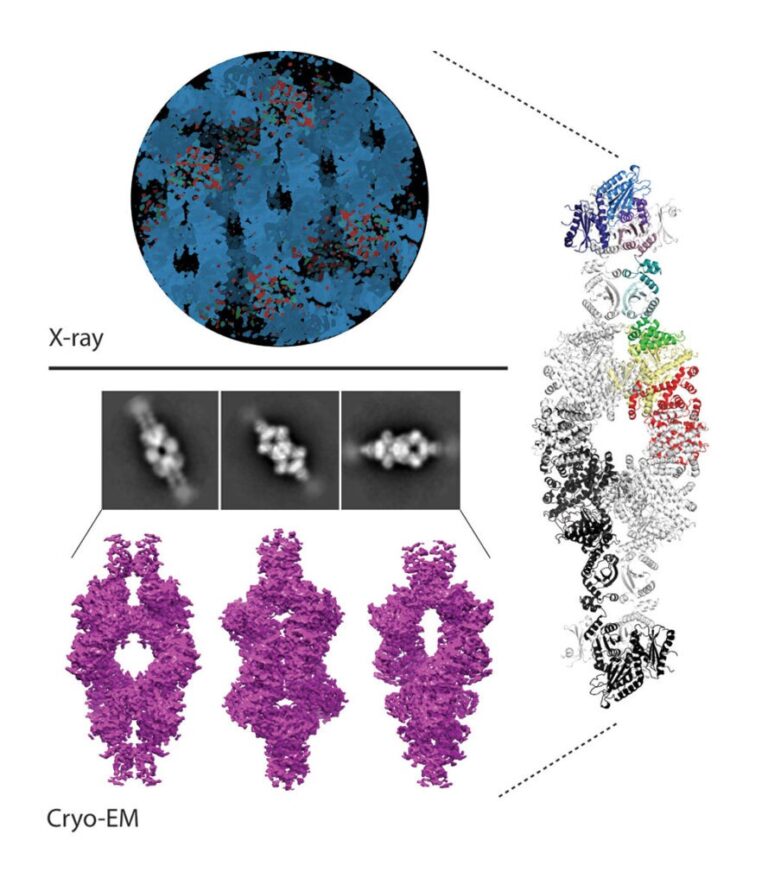
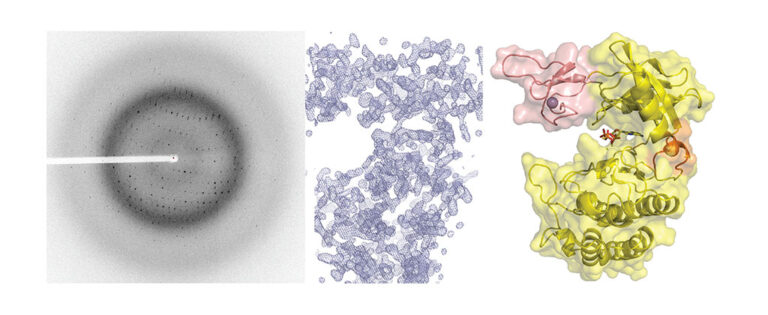
We have contributed to demonstrate that in diverse Actinobacteria, nitrogen metabolism is regulated by the signaling pathway involving the protein kinase PknG. In both the pathogen that causes tuberculosis in humans and in free-living species used in biotechnological processes, PknG controls intracellular glutamate levels depending on the amino acids present in the medium. Notably, the integrity of this signal transduction pathway is critical for the virulence of Koch bacillus. In Mycobacterium, the regulator GarA modulates, by direct interaction, three metabolic enzymes, including a high molecular weight glutamate dehydrogenase (L-GDH, L for large). This determines that alpha-ketoglutarate is diverted from the Krebs cycle in favor of glutamate synthesis; Instead, phosphorylation of GarA by PknG inhibits the regulator’s action, and alpha-ketoglutarate is then directed to the Krebs cycle. We recently elucidated the 3D structure of GarA-modulated mycobacterial L-GDH. These findings reveal unique aspects of the architecture of this type of enzyme and suggest novel regulatory mechanisms. A deep understanding of the molecular mechanisms involved in this signaling pathway will allow us to decipher how M. tuberculosis and other actinobacteria modulate their metabolism and other key physiological processes. This will contribute to the design of anti-tuberculosis drugs and biotechnological innovations.
SERVICES
Technological services are provided through the Argentine Platform for Structural Biology and Metabolomics (PLBEM).
Images of our research lines