research groups
Bacterial Sensors
summary

- Llarrull, Leticia Location: CCT
Email: llarrull@ibr-conicet.gov.ar
- Palanca, Ignacio
- Peralta, Melani
- Rodriguez Aieta, Constanza
- Cairoli, Julieta
RESEARCH LINES
Staphylococcus aureus is the main cause of intra- and extra-hospital infections in the world.
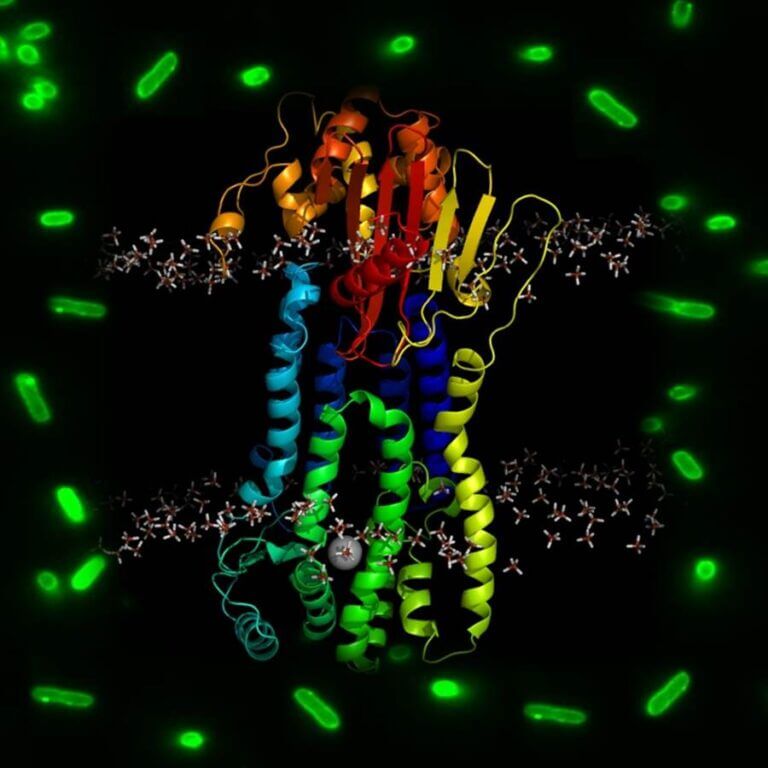
There is great concern about the increasing prevalence of infections caused by strains of S. aureus resistant to the action of β-lactam antibiotics (MRSA, methicillin-resistant S. aureus). The mechanisms of resistance to β-lactam antibiotics in S. aureus are activated only when the bacterium is in the presence of a β-lactam. This activation is regulated by the transmembrane sensor/signal transducer proteins BlaR1 and MecR1. MecR1 regulates the expression of the transpeptidase PBP2a, which is not inhibited by clinical concentrations of most of these antibiotics. While BlaR1 regulates the expression of the serin-β-lactamase PC1 and, in some strains, of PBP2a. Our group is focused on structurally characterizing MecR1 and BlaR1, to understand the conformational changes that lead to the activation of these metalloproteases by β-lactams. For this we combine microbiological (reporter strains), biochemical (activity assays, chemical crosslinking with photoactivatable compounds, among others) and biophysical (cryo-electron microscopy, circular dichroism, Electron Paramagnetic Resonance spectroscopy (in collaboration with Dr. Tabares, I2BC, France) and Luminescence Resonance Energy Transfer spectroscopy, among other techniques).
Molecular details of the recognition of β-lactam antibiotics and glycopeptides by the sensor proteins of the VraSRT system of Staphylococcus aureus
The glycopeptide vancomycin is the antibiotic available for the treatment of MRSA infections. However, the outlook is not encouraging, given that some strains of S. aureus have also developed mechanisms of resistance to vancomycin. The three-component system VraSRT coordinates the response to antibiotics that inhibit cell wall peptidoglycan biosynthesis in S. aureus and is associated with resistance to glycopeptide and β-lactam antibiotics. The inactivation of this system drastically affects the resistance of different strains of S. aureus to vancomycin and to β-lactam antibiotics. The signal that triggers the activation of the VraS sensor/kinase protein and the role of the VraT membrane protein are unknown. Using antibiotic-derived photoprobes, reporter strains, and kinase/phosphotransferase activity assays, we are evaluating whether the activation is due to direct antibiotic interaction. In addition, we address the structural characterization of the conformational changes that give rise to activation, using cryo-electron microscopy, circular dichroism, Electron Paramagnetic Resonance spectroscopy (in collaboration with Dr. Tabares, I2BC, France) and Luminescence Resonance Energy Transfer, among other techniques.
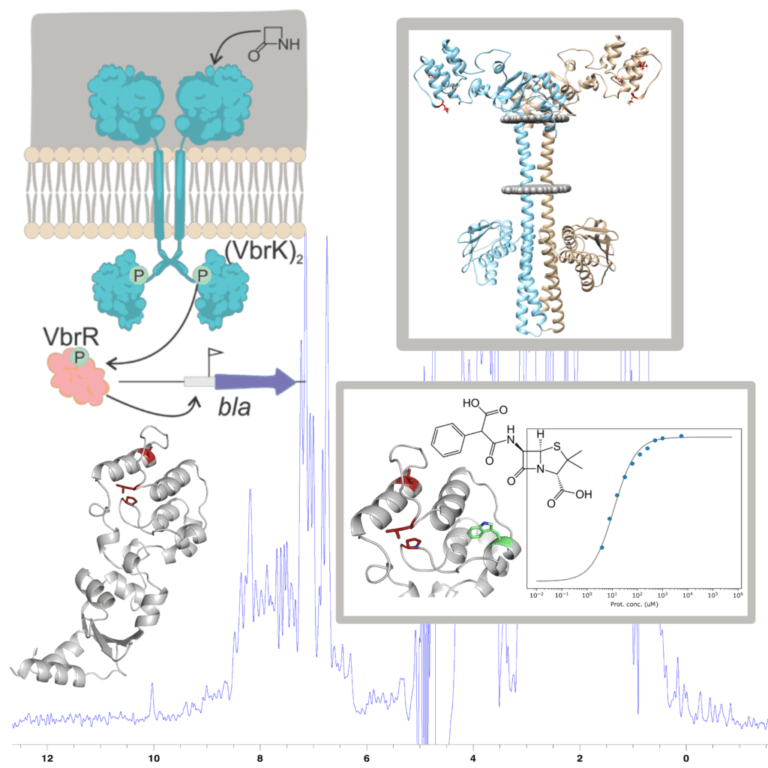
Recognition of β-lactam antibiotics by the VbrKR resistance system of Vibrio parahaemolyticus
The emergence of bacteria resistant to β-lactam antibiotics is a worldwide public health problem. Resistant strains of the genus Vibrio (causing acute intestinal infections) have been reported around the globe, posing a threat especially in developing countries. Recently, a new direct sensor system for β-lactam antibiotics that activates the production of β-lactamase has been described in V. parahaemolyticus. It is a two-component system called VbrKR. This system is mainly found in bacteria of the genus Vibrio, making it a specific target for the treatment of infections caused by species of this genus. Our goal is to understand the molecular activation mechanism of VbrKR. Using biophysical techniques, we aim to characterize the affinity of the receptor for different antibiotics, determine its structure by X-ray crystallography in the presence and absence of the antibiotic, determine its oligomeric state, and generate a system to map the interactions by NMR. This information will provide the basis for the rational drug design.
Identification of inhibitors of β-lactam antibiotic sensors in Staphylococcus aureus
Resistance to β-lactam and glycopeptide antibiotics in S. aureus is due to the inducible expression of different resistance determinants. S. aureus has membrane proteins that are sensors for these antibiotics. The sensor proteins can be metalloproteases (as is the case with the BlaR1 and MecR1 proteins) or kinases of two/three-component systems (as is the case with the VraS protein). The interaction with the antibiotic unleashes a signal transduction mechanism that leads to the activation of the cytoplasmic domain of these proteins (catalytic domain), giving rise to a cascade of reactions in the cytoplasm that culminates in the expression of resistance determinants. In collaboration with Dr. Rodriguez (IBR) and Dr. Testero (IQUIR) we are working to identify compounds capable of inhibiting the activation of these resistance systems and to characterize their inhibition mechanism. The finding of inhibitory compounds would provide key information to understand the mechanism of signal transduction. In addition, these inhibitors would allow the design of combined therapies with β-lactams/glycopeptides for the treatment of infections with resistant strains.
Images of our research lines
PUBLICATIONS AND PATENTS
Medicinal Chemistry of β-Lactam Antibiotics.
Medicinal Chemistry of β-Lactam Antibiotics.
An experiment-informed signal transduction model for the role of the Staphylococcus aureus MecR1 protein in β-lactam resistance.
An experiment-informed signal transduction model for the role of the Staphylococcus aureus MecR1 protein in β-lactam resistance.
Discovery of a New Class of Non-β-Lactam Inhibitors of Penicillin-binding Proteins with Gram-positive Antibacterial Activity.
Discovery of a New Class of Non-β-Lactam Inhibitors of Penicillin-binding Proteins with Gram-positive Antibacterial Activity.
How Allosteric Control of Staphylococcus aureus Penicillin-Binding Protein 2a Enables Methicillin-Resistance and Physiological Function.
How Allosteric Control of Staphylococcus aureus Penicillin-Binding Protein 2a Enables Methicillin-Resistance and Physiological Function.
The Histidine Kinase VraS Interacts with Vancomycin and PenicillinsThrough its Membrane-Anchored N-terminal Domain
The Histidine Kinase VraS Interacts with Vancomycin and PenicillinsThrough its Membrane-Anchored N-terminal Domain
Sede CCT Rosario
Ocampo y Esmeralda, Predio CONICET-Rosario
2000 Rosario, Santa Fe, Argentina
Tel. 54-341-4237070 / 4237500 / 4237200
Sede Facultad de Ciencias Bioquímicas y Farmacéuticas
Universidad Nacional de Rosario - Suipacha 531
2000 Rosario, Santa Fe, Argentina
Tel. +54 341 4350596 / 4350661 / 4351235
🔬 El IBR suma 9 proyectos seleccionados en Investigación Orientada 2025 de @ProduccionSF y @CienciaSantaFe.
Biotecnología, salud y sostenibilidad para fortalecer el vínculo entre ciencia, innovación y desarrollo territorial.

