research groups
Structural Microbiology and Biodesign lab
summary

- Lisa, María Natalia Location: CCT
Email: lisa@ibr-conicet.gov.ar
- Chamorro, Nicolas
- Rapino, Carina
- Azzolini, Juan Manuel
RESEARCH LINES
Molecular mechanisms of nitrogen metabolism regulation in Actinobacteria
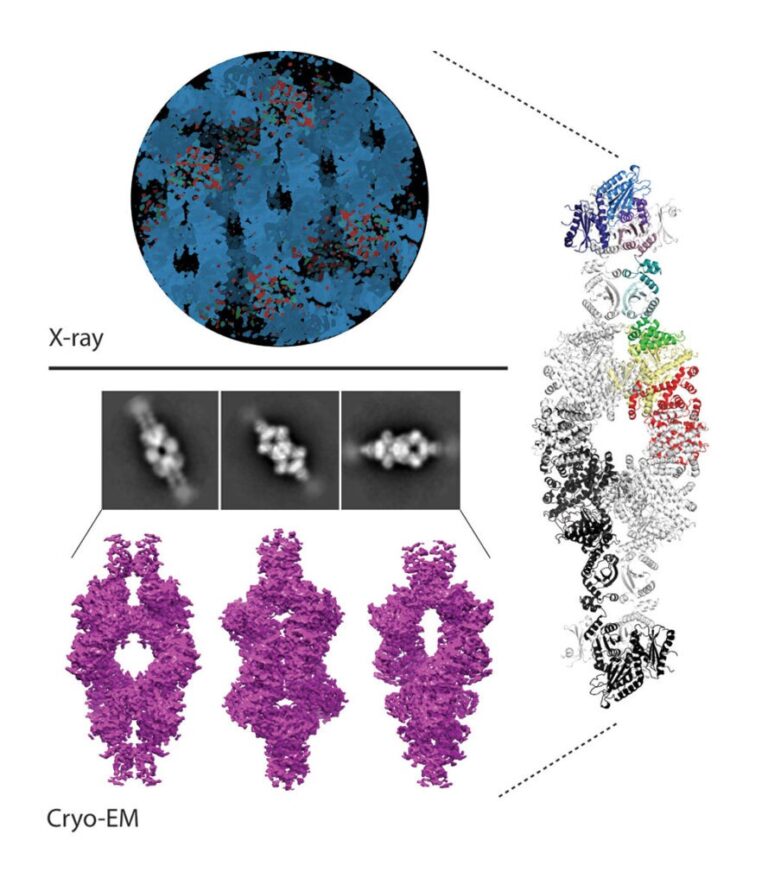
We have contributed to demonstrate that in diverse Actinobacteria, nitrogen metabolism is regulated by the signaling pathway involving the protein kinase PknG. In both the pathogen that causes tuberculosis in humans and in free-living species used in biotechnological processes, PknG controls intracellular glutamate levels depending on the amino acids present in the medium. Notably, the integrity of this signal transduction pathway is critical for the virulence of Koch bacillus. In Mycobacterium, the regulator GarA modulates, by direct interaction, three metabolic enzymes, including a high molecular weight glutamate dehydrogenase (L-GDH, L for large). This determines that alpha-ketoglutarate is diverted from the Krebs cycle in favor of glutamate synthesis; Instead, phosphorylation of GarA by PknG inhibits the regulator’s action, and alpha-ketoglutarate is then directed to the Krebs cycle. We recently elucidated the 3D structure of GarA-modulated mycobacterial L-GDH. These findings reveal unique aspects of the architecture of this type of enzyme and suggest novel regulatory mechanisms. A deep understanding of the molecular mechanisms involved in this signaling pathway will allow us to decipher how M. tuberculosis and other actinobacteria modulate their metabolism and other key physiological processes. This will contribute to the design of anti-tuberculosis drugs and biotechnological innovations.
SERVICES
Technological services are provided through the Argentine Platform for Structural Biology and Metabolomics (PLBEM).
Images of our research lines
PUBLICATIONS AND PATENTS
3D architecture and structural flexibility revealed in the subfamily of large glutamate dehydrogenases by a mycobacterial enzyme.
3D architecture and structural flexibility revealed in the subfamily of large glutamate dehydrogenases by a mycobacterial enzyme.
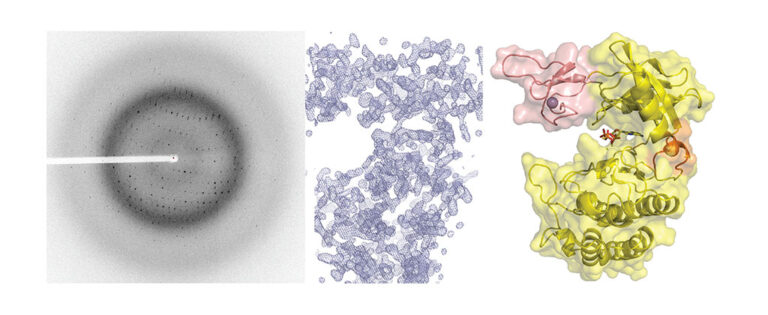
A Tetratricopeptide Repeat Scaffold Couples Signal Detection to OdhI Phosphorylation in Metabolic Control by the Protein Kinase PknG.
A Tetratricopeptide Repeat Scaffold Couples Signal Detection to OdhI Phosphorylation in Metabolic Control by the Protein Kinase PknG.
PknG senses amino acid availability to control metabolism and virulence of Mycobacterium tuberculosis.
PknG senses amino acid availability to control metabolism and virulence of Mycobacterium tuberculosis.
Molecular Basis of the Activity and the Regulation of the Eukaryotic-like S/T Protein Kinase PknG from Mycobacterium tuberculosis.
Molecular Basis of the Activity and the Regulation of the Eukaryotic-like S/T Protein Kinase PknG from Mycobacterium tuberculosis.
Sede CCT Rosario
Ocampo y Esmeralda, Predio CONICET-Rosario
2000 Rosario, Santa Fe, Argentina
Tel. 54-341-4237070 / 4237500 / 4237200
Sede Facultad de Ciencias Bioquímicas y Farmacéuticas
Universidad Nacional de Rosario - Suipacha 531
2000 Rosario, Santa Fe, Argentina
Tel. +54 341 4350596 / 4350661 / 4351235
🔬 El IBR suma 9 proyectos seleccionados en Investigación Orientada 2025 de @ProduccionSF y @CienciaSantaFe.
Biotecnología, salud y sostenibilidad para fortalecer el vínculo entre ciencia, innovación y desarrollo territorial.

