research groups
Structure and Physiology of Microbial Biofilms Lab
summary

- Serra, Diego Location: CCT
Email: dserra@ibr-conicet.gov.ar
- Obando, Mayra
- Valentinis Rossi, Franco
- Abitante, Alma
- Olazaran Mainetti, Josefina
RESEARCH LINES
Spatial patterns and mechanisms of antibiotic tolerance in bacterial biofilms
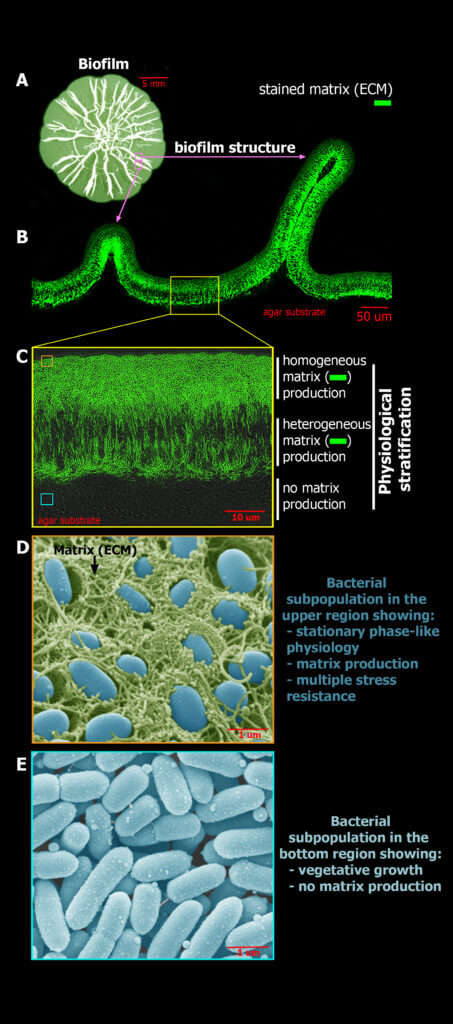
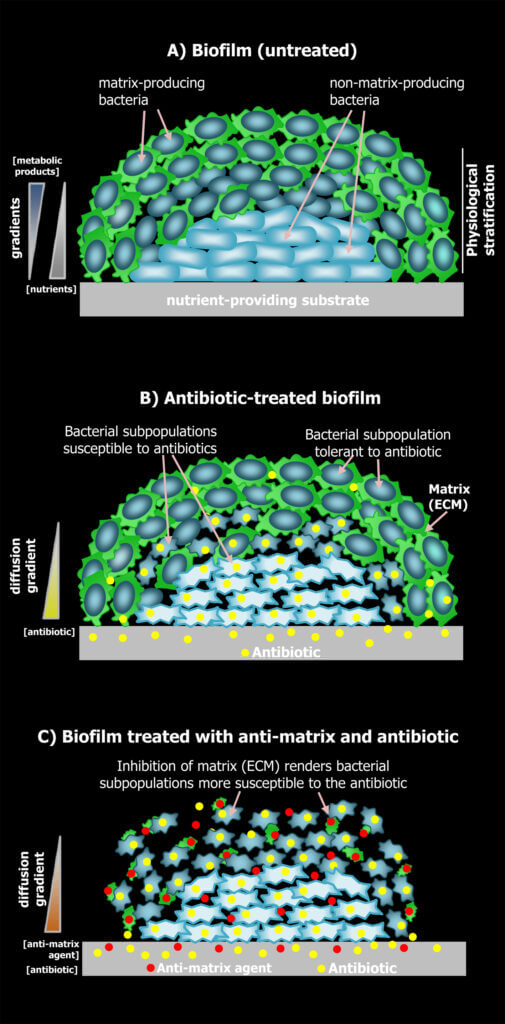
Due to their structural complexity, biofilms exhibit highly heterogeneous internal microenvironments shaped by gradients of nutrients, metabolic products and signalling compounds. Here, bacterial cells essentially adjust their physiology according to the local conditions, which ultimately causes the stratification of the biofilms into physiologically distinct regions. Our work suggests that this physiological stratification not only represents a division of labor, where cell subpopulations locally specialize in fulfilling specific tasks, such as for example the production of extracellular matrix, but it also endows cell subpopulations with different capacities to cope with stresses as it is the case of antibiotic treatments. Our group is interested in characterizing this physiological stratification in biofilms of commensal and pathogenic E. coli strains and in understanding how this physiological heterogeneity influences the chances of cells to survive antibiotic treatments depending on their location within the biofilm. In particular, we seek to clarify how heterogeneity in the production of extracellular matrix components (amyloid curli and pEtN-cellulose), in stress responses, and in metabolism/growth among cell subpopulations influence antibiotic tolerance in E. coli biofilms. Knowledge of these aspects is crucial to design therapies that can target all cell subpopulations that coexist within these communities, irrespective of their physiological state and spatial location.
Identification and mechanisms of action of anti-biofilm compounds
Recognizing the need for solutions to combat biofilm-based infections, we are also interested in discovering anti-biofilm compounds and characterizing their molecular mechanisms of action. In particular, we search for compounds that can interfere with the production of amyloid curli and pEtN-cellulose, the major extracellular matrix components, essential for the structural development of E. coli biofilms. As a platform for the search of inhibitors we are exploring microbial interactions in biofilms. While antagonistic interactions among microorganisms have been intensely exploited in the search for antibiotics, i.e., compounds that directly kill or inhibit bacterial growth, these interactions have been overlooked regarding their potential as sources for compounds that, rather than killing the bacteria, modulate or interfere with other bacterial behaviours such as the formation of biofilms. The use of anti-biofilm compounds is crucial in the fight to eradicate biofilm-based infections as they can considerably increase the effectiveness of antibiotics in combined therapies or enhance the efficacy of clearance by the host immune system.
Images of our research lines
PUBLICATIONS AND PATENTS
Bacterial multicellularity: the biology of Escherichia coli building large-scale biofilm communities.
Bacterial multicellularity: the biology of Escherichia coli building large-scale biofilm communities.
A c-di-GMP-based switch controls local heterogeneity of extracellular matrix synthesis which is crucial for integrity and morphogenesis of Escherichia coli macrocolony biofilms.
A c-di-GMP-based switch controls local heterogeneity of extracellular matrix synthesis which is crucial for integrity and morphogenesis of Escherichia coli macrocolony biofilms.
Spatial organisation of different sigma factor activities and c-di-GMP signalling within the 3D landscape of a bacterial biofilm.
Spatial organisation of different sigma factor activities and c-di-GMP signalling within the 3D landscape of a bacterial biofilm.
Phosphoethanolamine cellulose: a naturally produced chemically modified cellulose.
Phosphoethanolamine cellulose: a naturally produced chemically modified cellulose.
The green tea polyphenol EGCG inhibits Escherichia coli biofilm formation by impairing amyloid curli fibre assembly and down-regulating the biofilm regulator CsgD via the σE-dependent sRNA RybB.
The green tea polyphenol EGCG inhibits Escherichia coli biofilm formation by impairing amyloid curli fibre assembly and down-regulating the biofilm regulator CsgD via the σE-dependent sRNA RybB.
Sede CCT Rosario
Ocampo y Esmeralda, Predio CONICET-Rosario
2000 Rosario, Santa Fe, Argentina
Tel. 54-341-4237070 / 4237500 / 4237200
Sede Facultad de Ciencias Bioquímicas y Farmacéuticas
Universidad Nacional de Rosario - Suipacha 531
2000 Rosario, Santa Fe, Argentina
Tel. +54 341 4350596 / 4350661 / 4351235
🔬 El IBR suma 9 proyectos seleccionados en Investigación Orientada 2025 de @ProduccionSF y @CienciaSantaFe.
Biotecnología, salud y sostenibilidad para fortalecer el vínculo entre ciencia, innovación y desarrollo territorial.

