Other Research
Gene Expression Regulation in Craniofacial Morphogenesis
summary
One-third of human birth defects affect the head and face. Many of these problems (such as cleft lip and palate) originate in the early stages of embryonic development, when a special group of cells called the cranial neural crest fail to form or function properly. In order to develop therapies that prevent or minimize these defects, it is crucial to understand the etiology and pathogenesis of the malformation syndromes. This requires a detailed knowledge of the developmental events that induce neural crest cells to form, maintain their survival, guide their migration, and influence their differentiation in the context of normal craniofacial development.
We use biochemical, molecular, biophysical, and bioinformatic tools to understand the gene regulation processes that underlie both normal development and anomalies. The zebrafish is our experimental model due to its genetic similarity to humans (over 70%), the transparency of its embryos, its rapid reproduction and development, making it a key model for studying human pathologies.

Sede:
Email:
RESEARCH LINES
CNBP as a regulator of craniofacial morphogenesis: a potential therapeutic target for neurocristopathies.
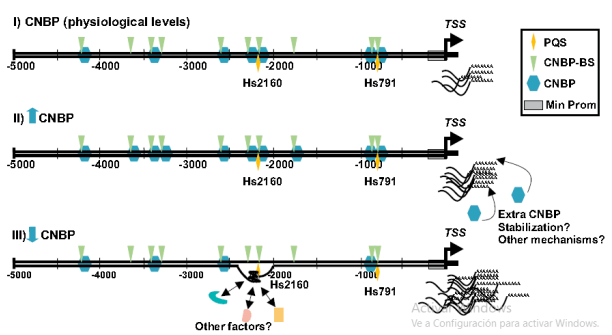
The pathology known as Treacher Collins Syndrome (TCS) is a hereditary mandibulofacial dysostosis caused primarily by mutations in the TCOF1 gene. Recently, we have established an association between the expression of CNBP (a modulator of craniofacial development sensitive to reactive oxygen species and susceptible to phosphorylation-regulated degradation via proteasome) and that of TCOF1. In addition, we have found that CNBP interacts with structural elements known as guanine quadruplexes (G4) in the TCOF1 promoters and is capable of modulating the expression of this gene in both human cells and zebrafish embryos. Understanding these regulatory mechanisms allows testing the manipulation of these phenomena with specific drugs (e.g. antioxidants, CNBP phosphorylation inducers). On the other hand, G4 folding can be stabilized or destabilized by specific ligands. This project proposes to explore these options as chemotherapeutic strategies using a TCS model developed in zebrafish.
CNBP as a regulator of craniofacial morphogenesis: expanding the network.
Our evidence suggests that CNBP could be a central regulator in a network involving G-quadruplex (G4) and other genes central to head development. We therefore propose a bioinformatics analysis of data available in various publicly available databases in order to measure the scope of this possible regulatory network and understand its mechanisms. The zebrafish model is the complement to validate our hypotheses and test the functioning of these regulatory networks in physiological and, eventually, pathological situations.
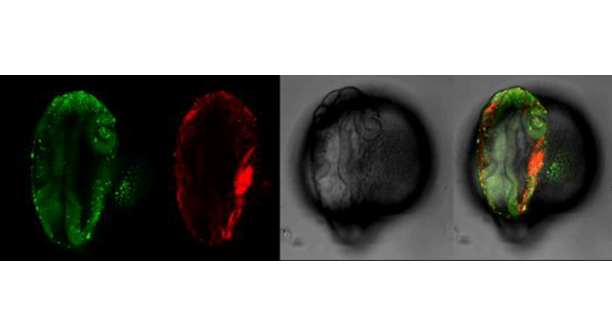
Images of our research lines
Links:
https://www.conicet.gov.ar/new_scp/detalle.php?id=31158&keywords=coux&datos_academicos=yes
https://www.researchgate.net/profile/Gabriela-Coux
Redes sociales
Twitter: @gcoux
